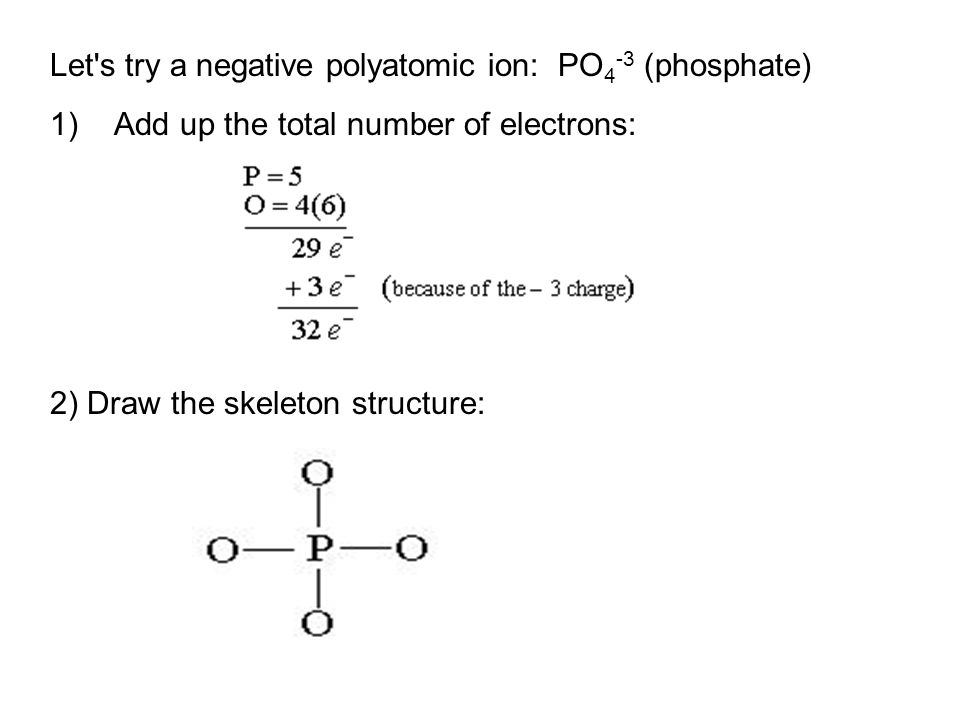
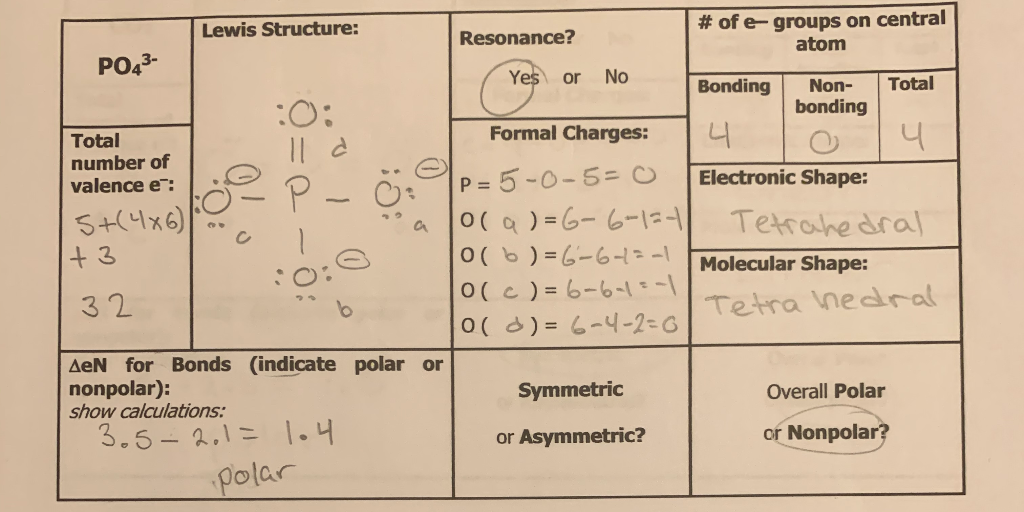
![SPOILER] [AAMC Official Guide Questions C/P Question #16] Is PO4(3-) polar or non-polar? EK says it is insoluble... AAMC says otherwise : r/Mcat SPOILER] [AAMC Official Guide Questions C/P Question #16] Is PO4(3-) polar or non-polar? EK says it is insoluble... AAMC says otherwise : r/Mcat](https://preview.redd.it/5whv97g4py811.png?auto=webp&s=31d1a7087b06684ca368313115093927e6bf433d)
SPOILER] [AAMC Official Guide Questions C/P Question #16] Is PO4(3-) polar or non-polar? EK says it is insoluble... AAMC says otherwise : r/Mcat

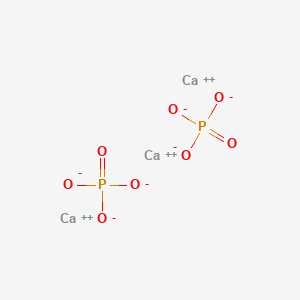
PO4 3- Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts – Lambda Geeks

PO4 3- Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts – Lambda Geeks

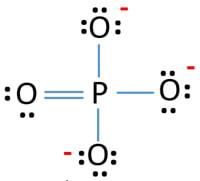
Draw the Lewis structure for PO43- and determine its electron and molecular geometries. | Homework.Study.com

PO4 3- Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts – Lambda Geeks

PO43- Lewis Structure (Phosphate Ion) | PO43- Lewis Structure (Phosphate Ion) Did you know that Phosphorus can have expanded orbitals and can accommodate more than 8 electrons in its outer... | By

PO4 3- Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts – Lambda Geeks

PO4 3- Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and Detailed Facts – Lambda Geeks