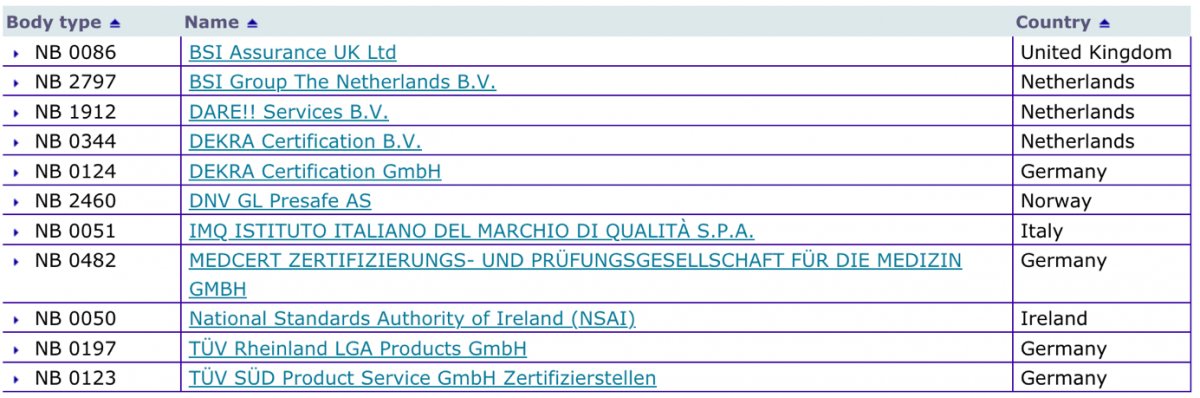
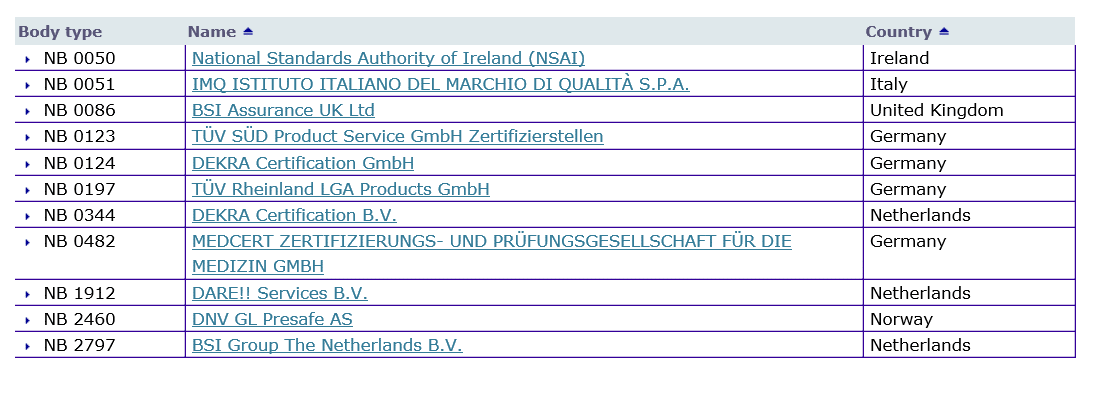
Low rate of Notified Body designations under MDR and IVDR causes bottleneck concerns for the European MedTech Industry

MDR: 26 Notified Bodies on NANDO & Swiss economic operator's requirements updated! · MDlaw – Information platform on European medical device regulations