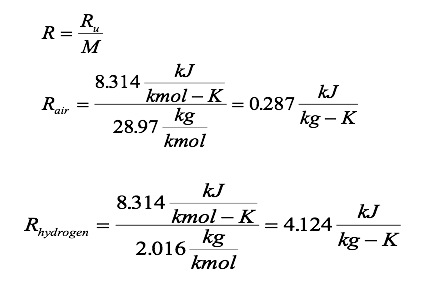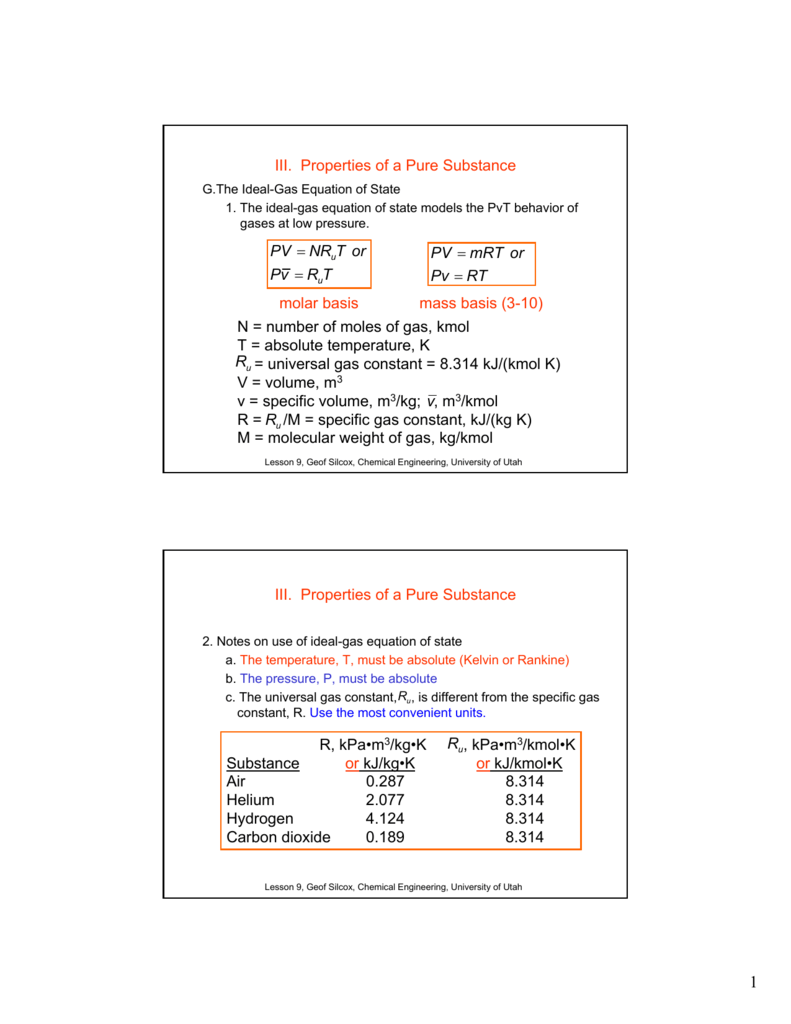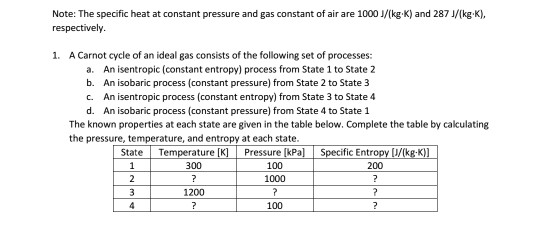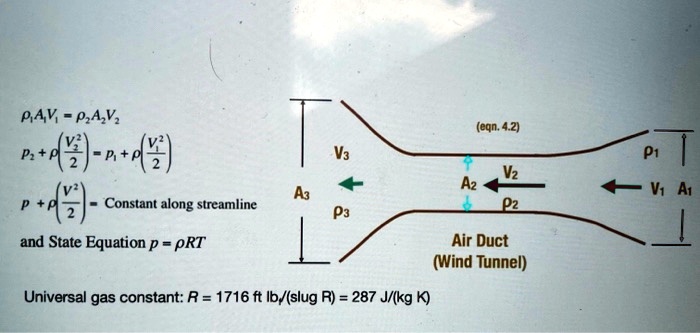
SOLVED:PAV PzA,Vz A+4)-a+4r) (egn. 4.2) Va Vz Az Pz Constant along streamline and State Equation p = pRT Air Duct (Wind Tunnel) Universal gas constant: R = 1716 ft Ib/(slug R) =

ExxonMobil Careers - Mechanical engineering students, it's #MechMondays! Every Monday, we post a question that will allow you to refresh your academic memory and demonstrate what you've learned in your engineering courses.

AOS 101 February 12 or 14 Ideal Gas Law. P = pressure (in Pascals) ρ = density (in kg/m 3 ) = mass / volume R = gas constant (dry air: R = 287 J/kg K) - ppt download

Polynomial coefficients representing gamma The specific gas constants... | Download Scientific Diagram
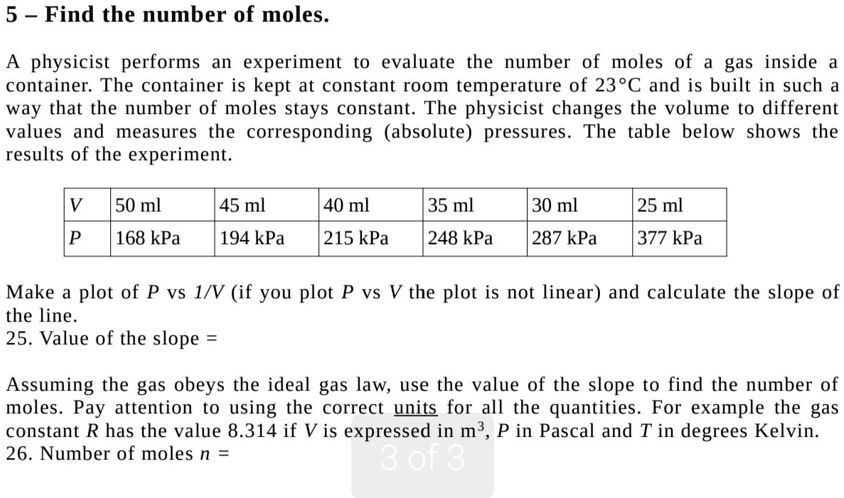
SOLVED:5 Find the number of moles. physicist performs an experiment to evaluate the number of moles of gas inside container: The container is kept at constant room temperature of 23*C and is


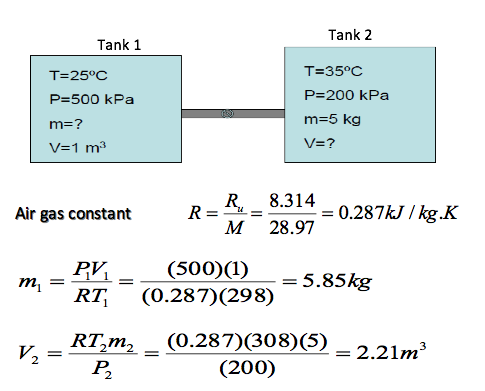

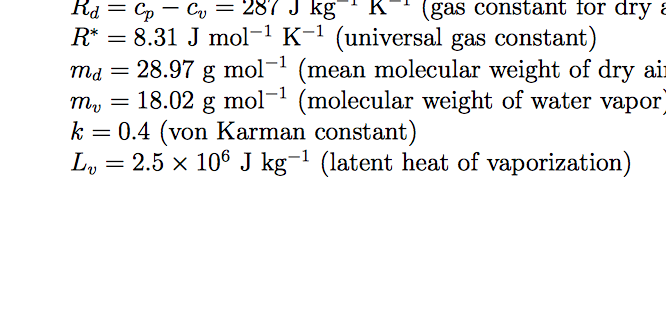

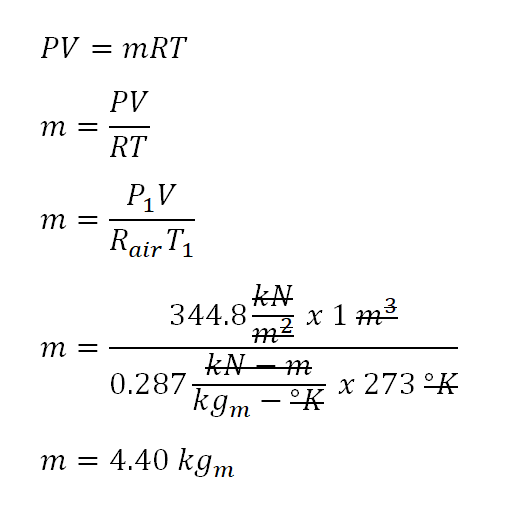

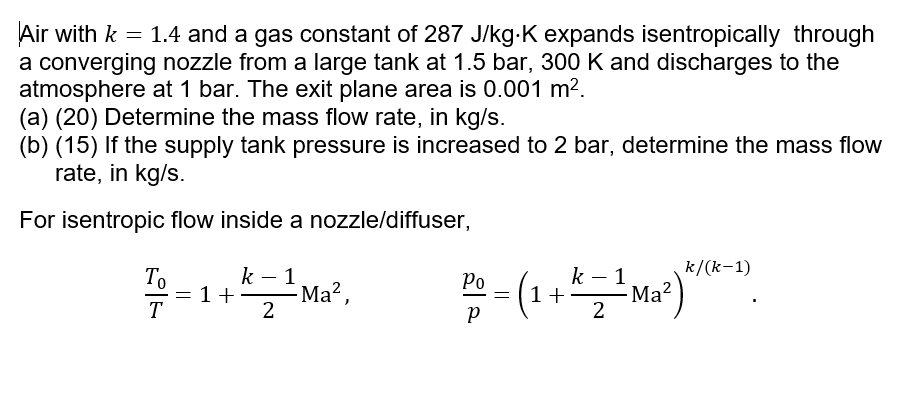




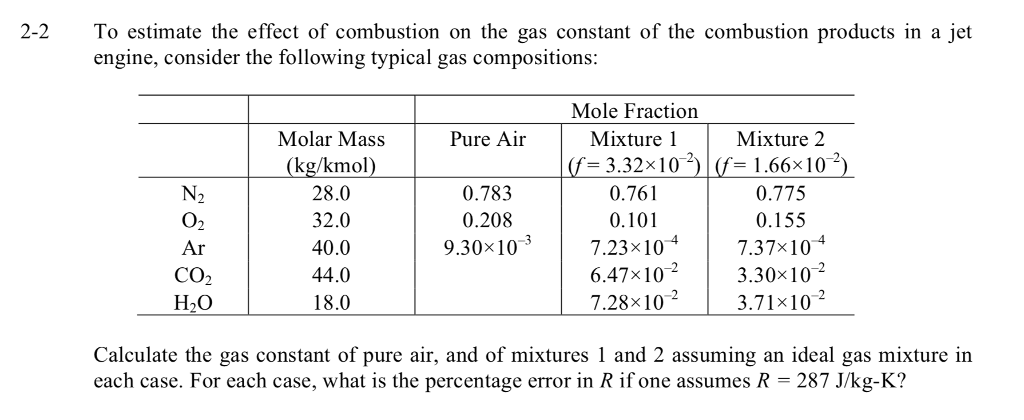
![Solved Air has a gas constant of R = 287[kg-K 287[kk]. At | Chegg.com Solved Air has a gas constant of R = 287[kg-K 287[kk]. At | Chegg.com](https://media.cheggcdn.com/media/764/764373f3-5d91-4e8f-92aa-13a05953de86/phpD4qEs7)