Global Clinical Trial Data Sharing - The Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard
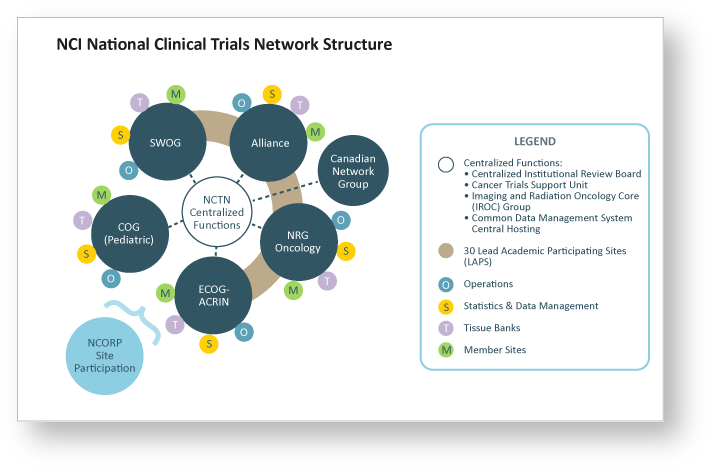
Imaging Clinical Trials - The Cancer Imaging Archive (TCIA) Public Access - Cancer Imaging Archive Wiki

Global Clinical Trial Data Sharing - The Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard
Reflections on Sharing Clinical Trial Data: Challenges and a Way Forward: Proceedings of a Workshop |The National Academies Press

Global Clinical Trial Data Sharing - The Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard

Sharing and reuse of individual participant data from clinical trials: principles and recommendations | BMJ Open

Open Data Revolution in Clinical Research: Opportunities and Challenges - Shahin - 2020 - Clinical and Translational Science - Wiley Online Library
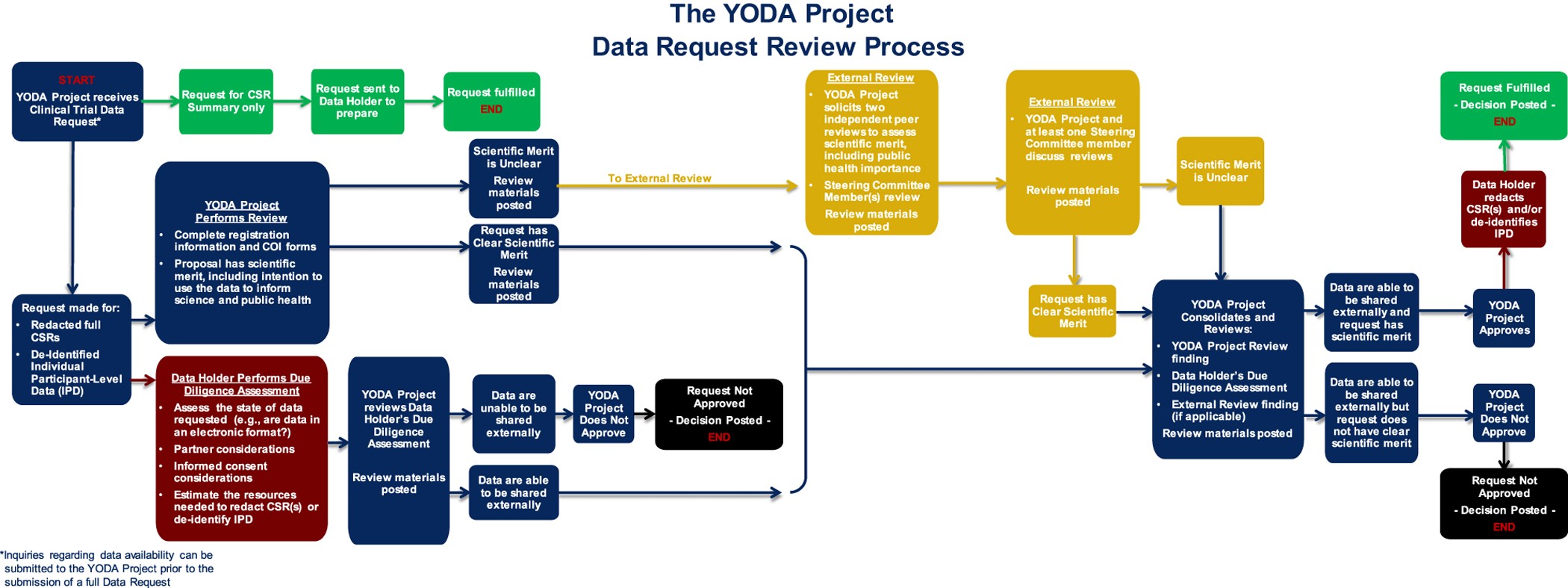
Overview and experience of the YODA Project with clinical trial data sharing after 5 years | Scientific Data

Data Sharing Statements for Clinical Trials — A Requirement of the International Committee of Medical Journal Editors | NEJM