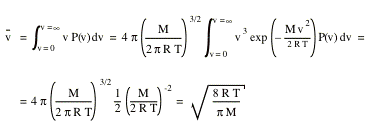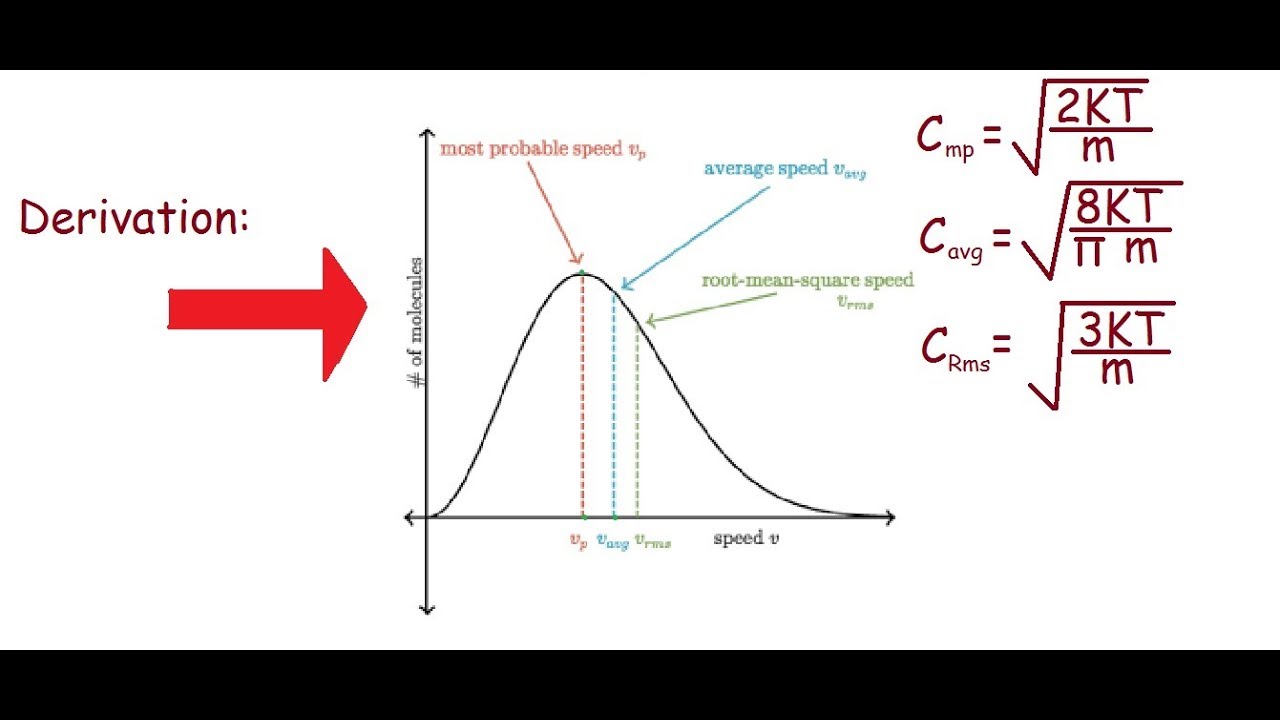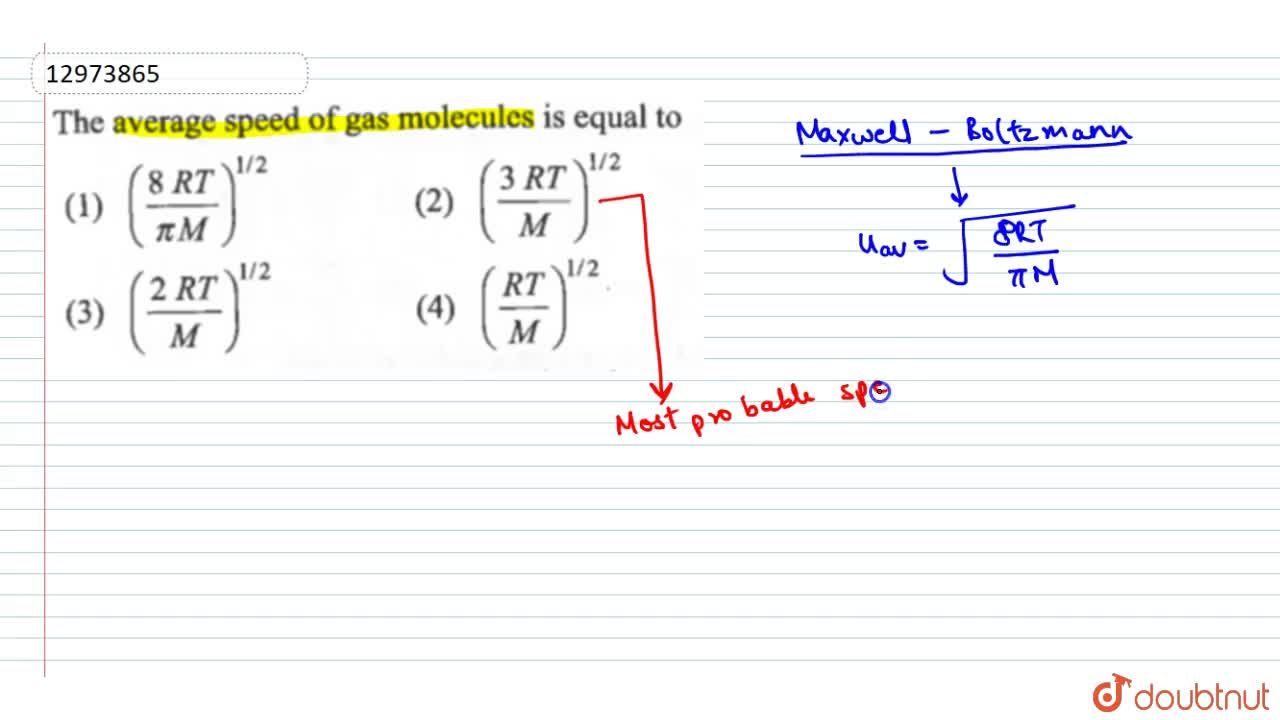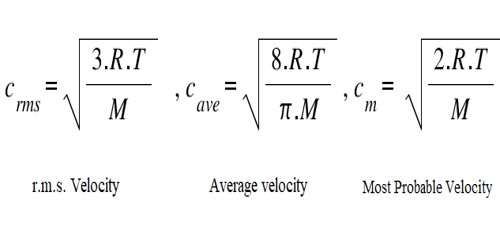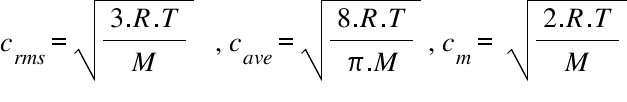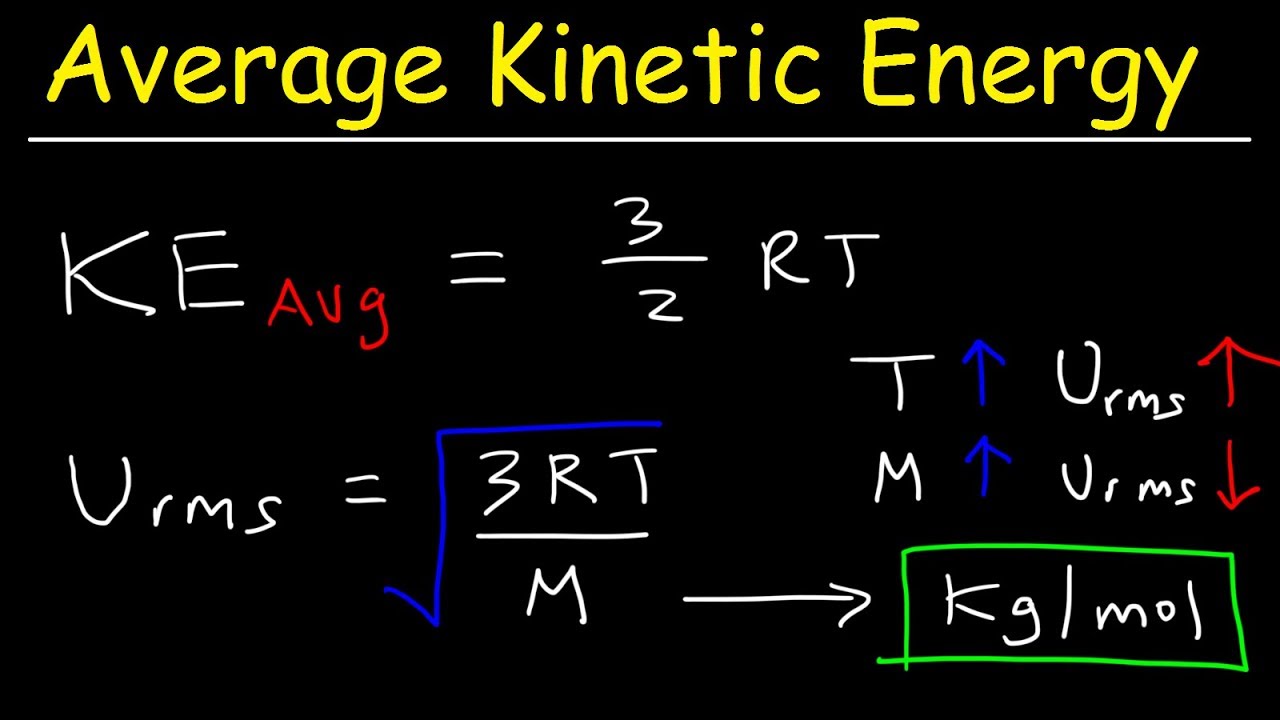
Average Kinetic Energy of a Gas and Root Mean Square Velocity Practice Problems - Chemistry Gas Laws - YouTube

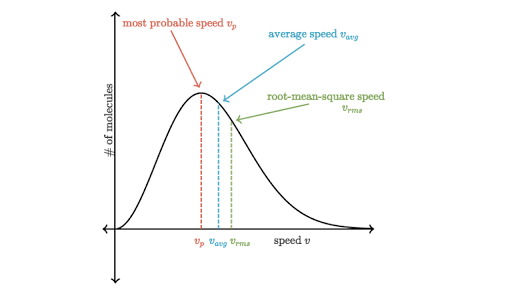
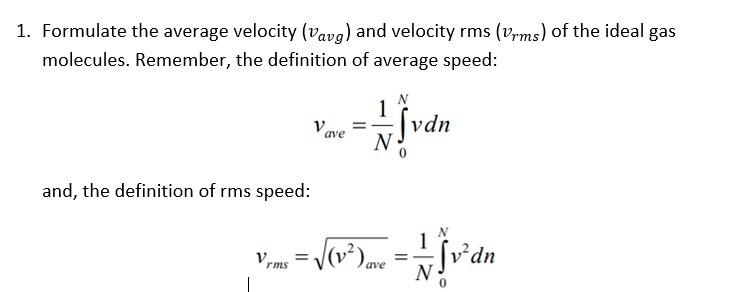
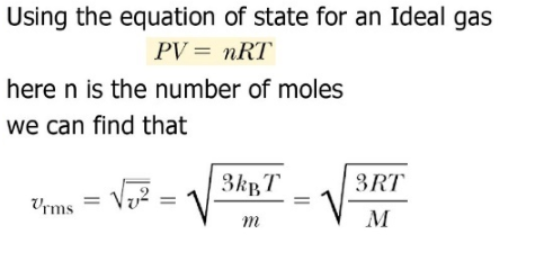
The average velocity of gas molecules is 400 m/sec . Calculate its rms velocity at the same temperature.
The average velocity of gas molecules is 400 m/s. Calculate its rms velocity at the same temperature. - Sarthaks eConnect | Largest Online Education Community
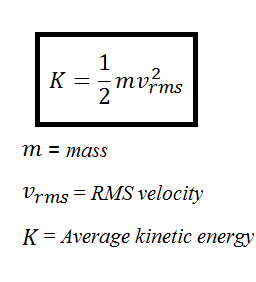
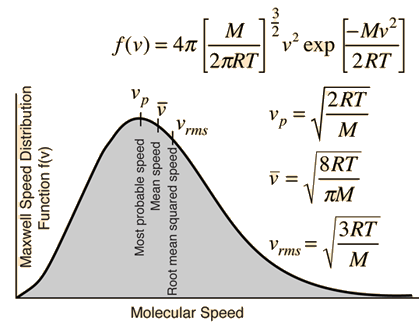
Kinetic Molecular Theory of Gases | Speed, Formula & Calculation - Video & Lesson Transcript | Study.com
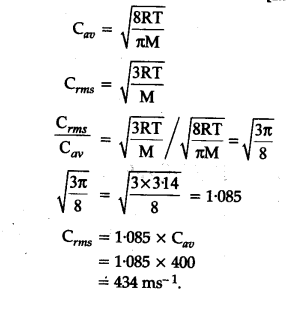
The average velocity of gas molecules is 400 m${{s}^{-1}}$.Calculate its r.m.s. velocity at the same temperature - CBSE Class 11 Chemistry - Learn CBSE Forum

At what temperature, the average speed of gas molecules be double of that at temperature, `27^(@)C`? - YouTube

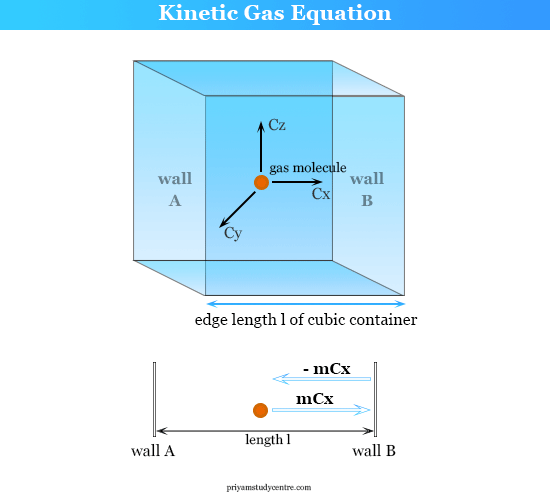
What is average velocity of molecules of gas of molecular weight M at temperature T - Chemistry - Some Basic Concepts of Chemistry - 14208519 | Meritnation.com