Hurdles in clinical implementation of academic advanced therapy medicinal products: A national evaluation - Cytotherapy

Clinical Development of Advanced Therapy Medicinal Products in Europe: Evidence That Regulators Must Be Proactive: Molecular Therapy
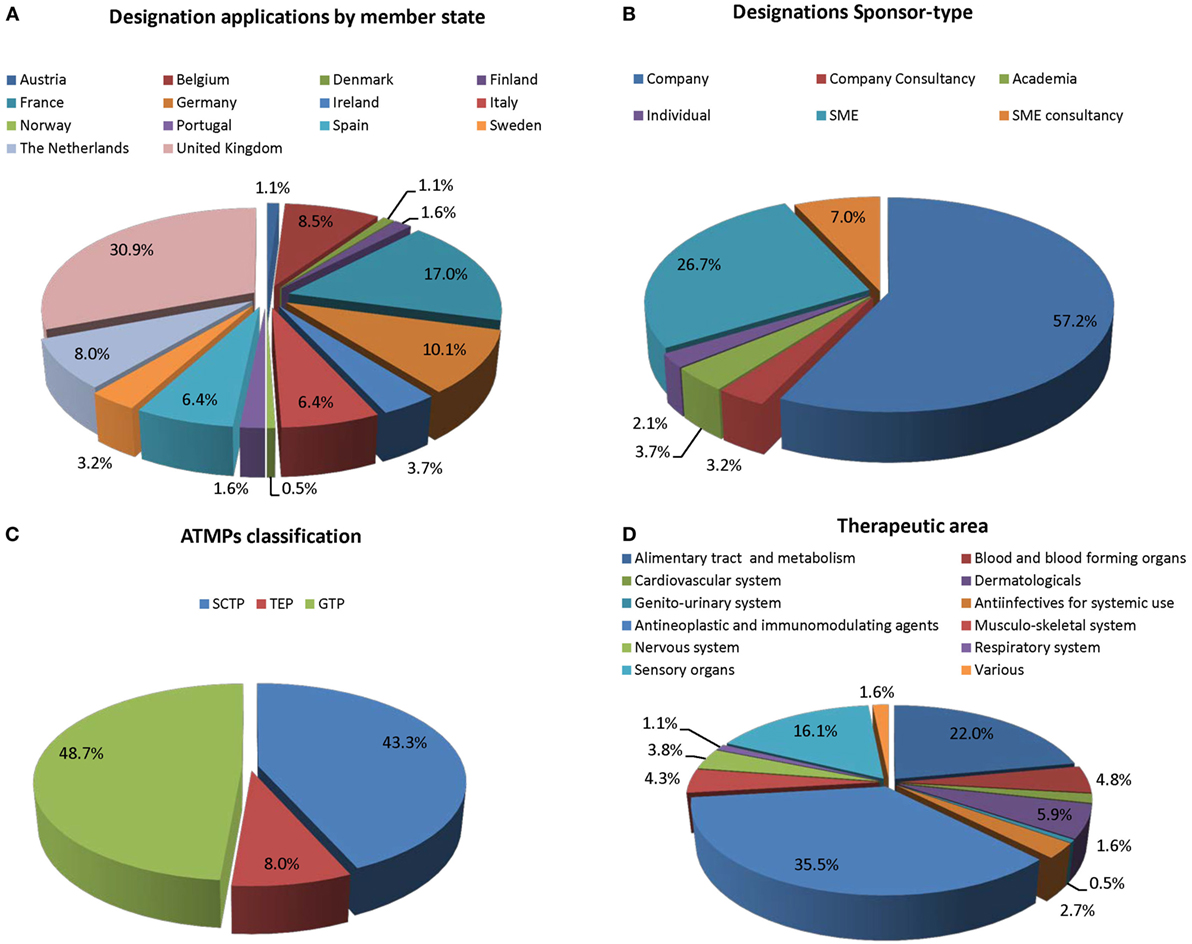
Frontiers | Advanced Therapy Medicinal Products for Rare Diseases: State of Play of Incentives Supporting Development in Europe

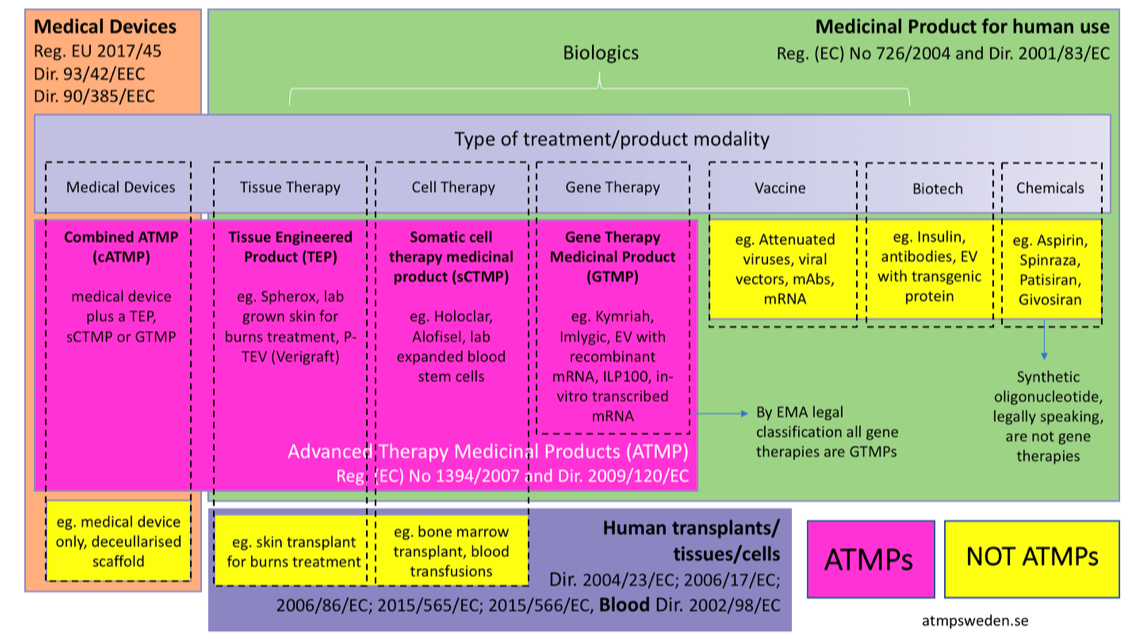
Cell-based product classification procedure: What can be done differently to improve decisions on borderline products? - ScienceDirect

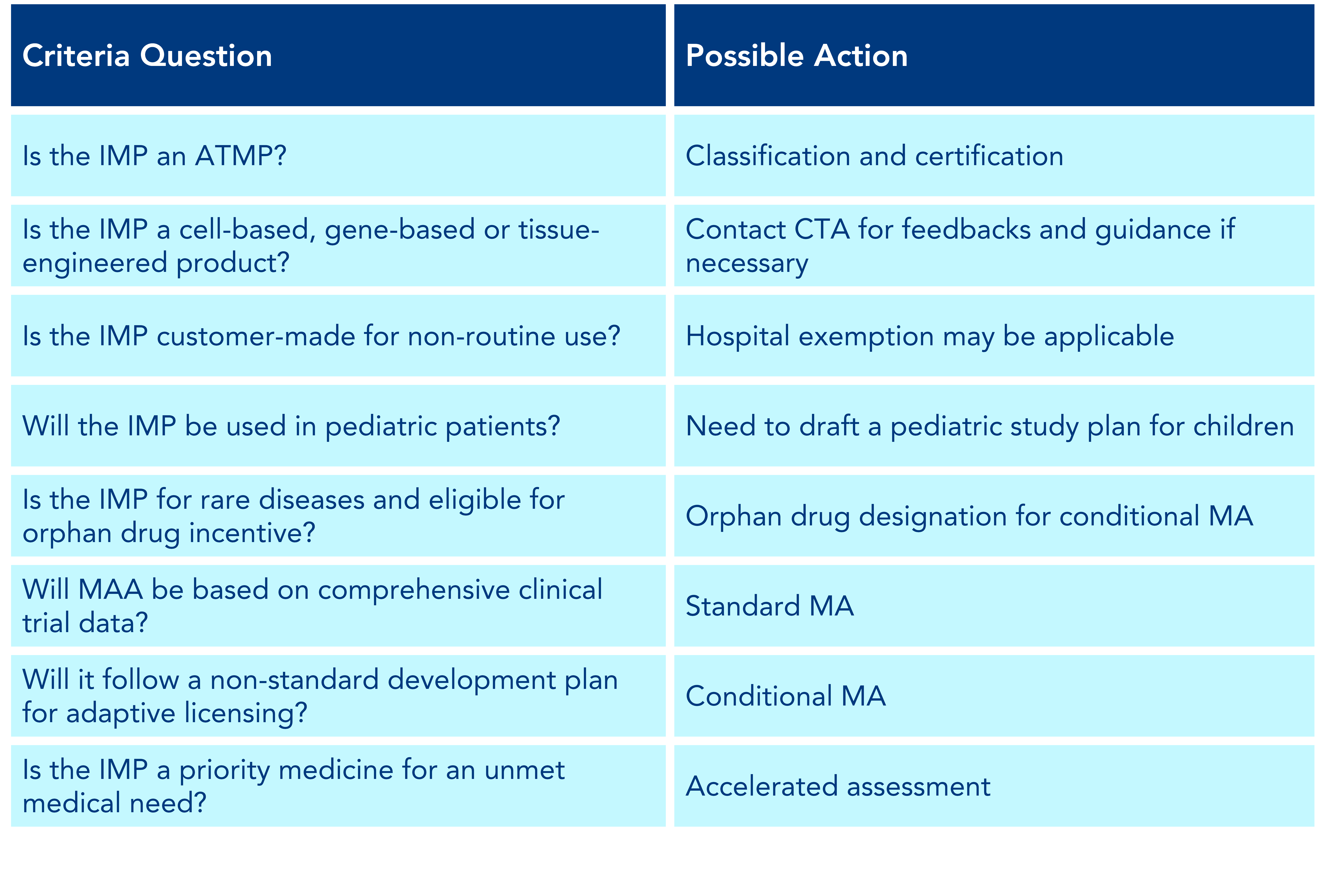
EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy - Methods & Clinical Development

Commercialisation of Advanced Therapies : - A Study of the EU Regulation on Advanced Therapy Medical Products | Semantic Scholar
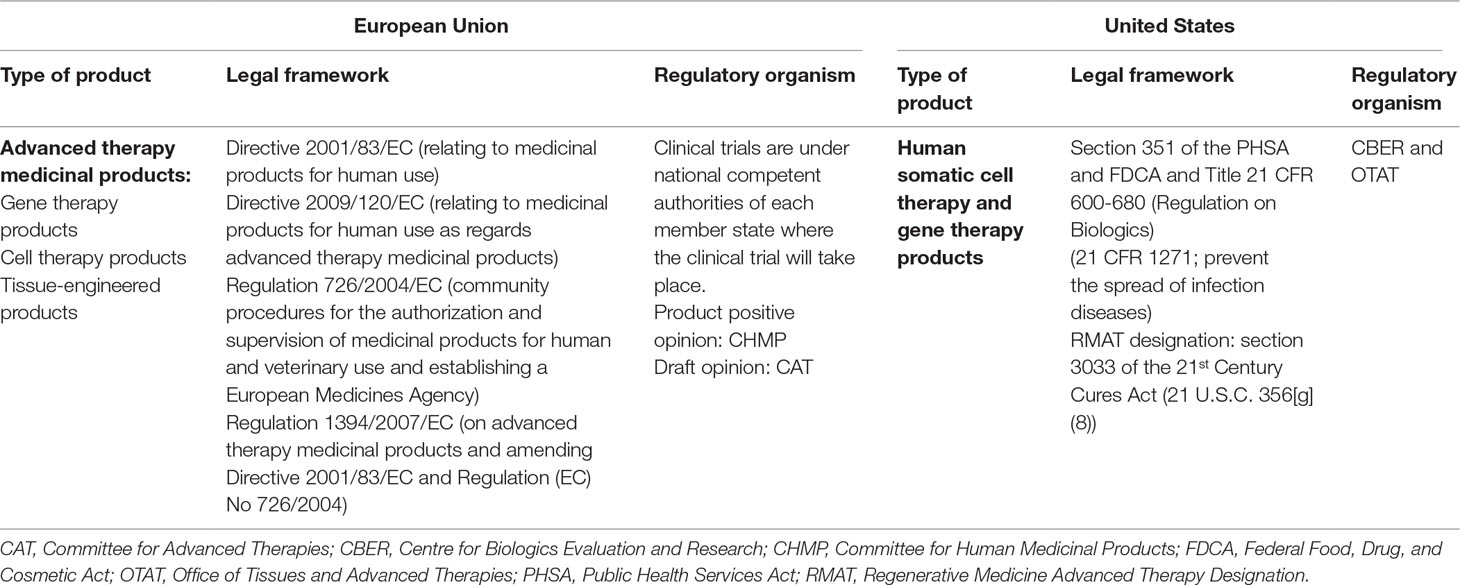
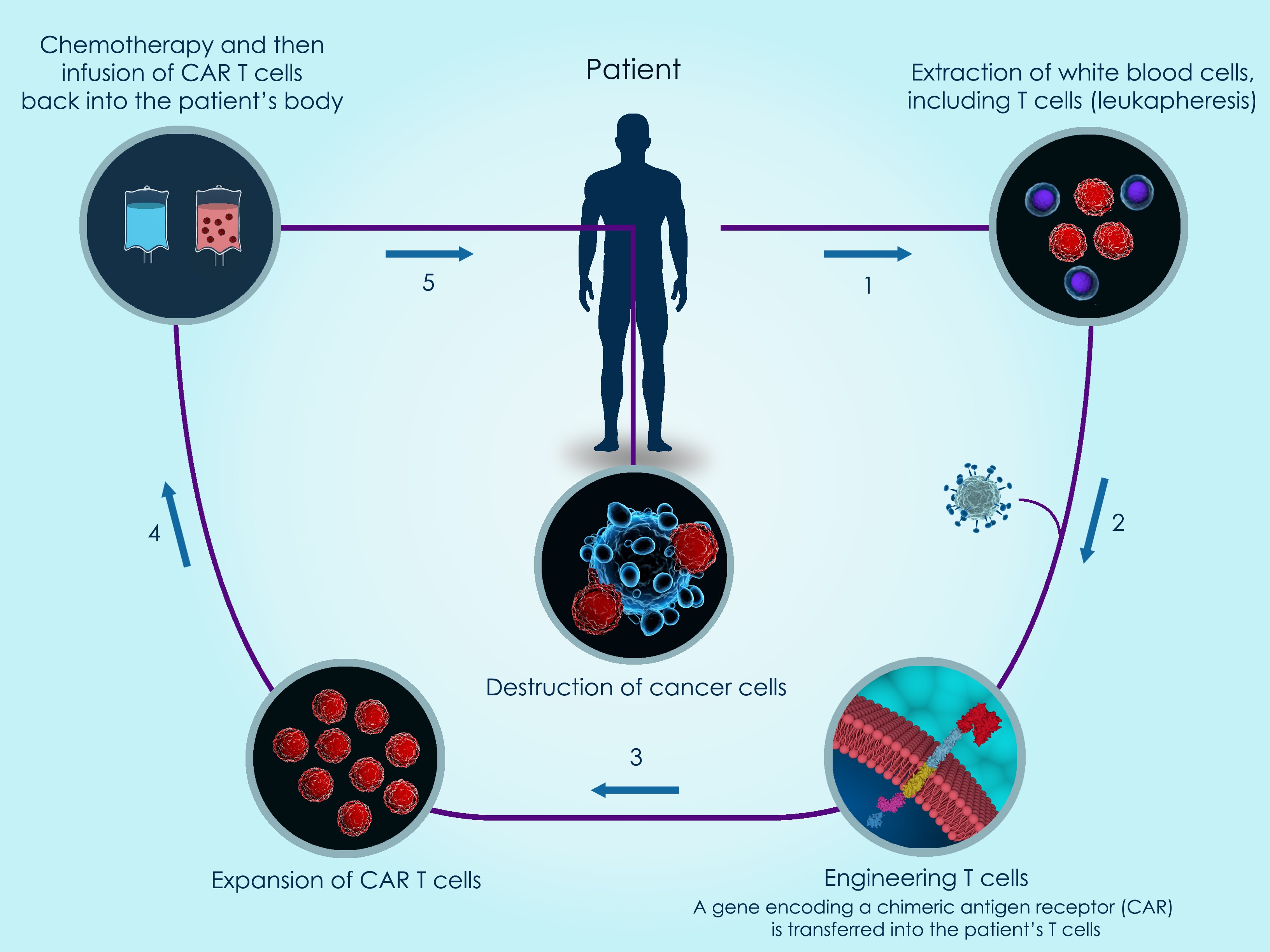
Frontiers | Regulatory Framework for Advanced Therapy Medicinal Products in Europe and United States

Clinical Development of Advanced Therapy Medicinal Products in Europe: Evidence That Regulators Must Be Proactive: Molecular Therapy

CELTIC-19 Granted Advanced Therapy Medicinal Product Classification by European Medicines Agency | Business Wire

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations